Blog

A new take on developing drugs for chronic pain
Despite a recent failure, Lilly’s master protocol trial shows a path forward in chronic pain R&D.

Red flags in biotech press releases
Learn the classic signs of spin and misdirection in biotech PR about clinical trial results.
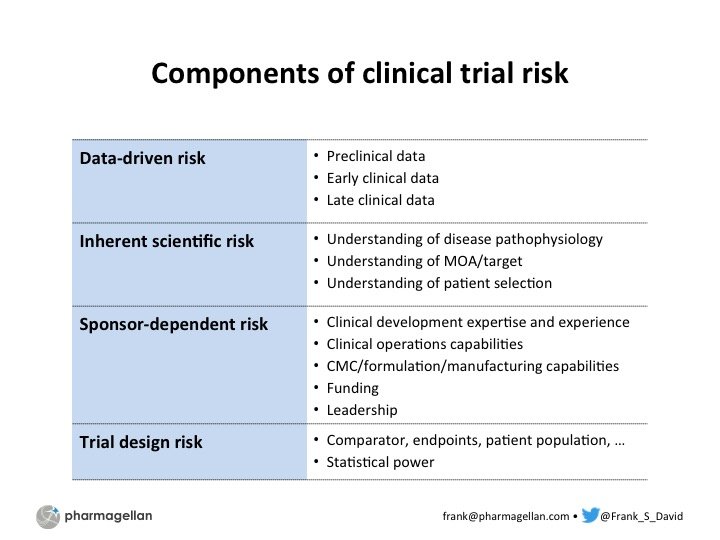
How risky is that biotech trial?
A framework to more methodically assess clinical development probability of success (POS).
Introductory resources for analyzing biotech clinical trial results (updated 3/28/24)
Learn to analyze biotech trials like a pro!
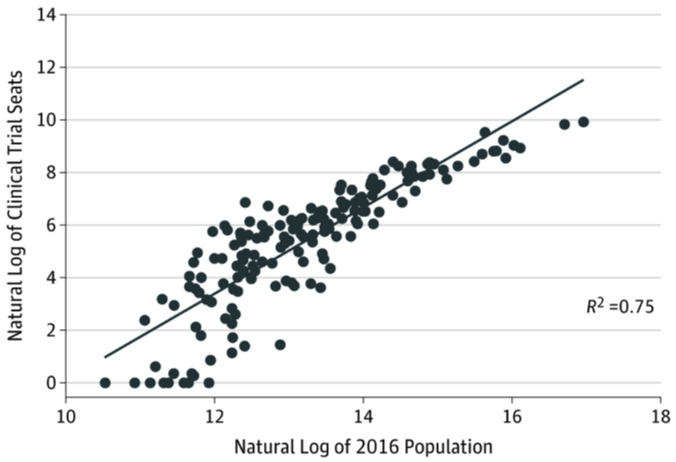
Does Boston have too many clinical trials?
Our data shed light on disparities in clinical study access across U.S. urban areas.

A biotech VC’s prescription for what ails pharma
How can we balance innovation and affordability in prescription drugs? Peter Kolchinsky has some ideas.
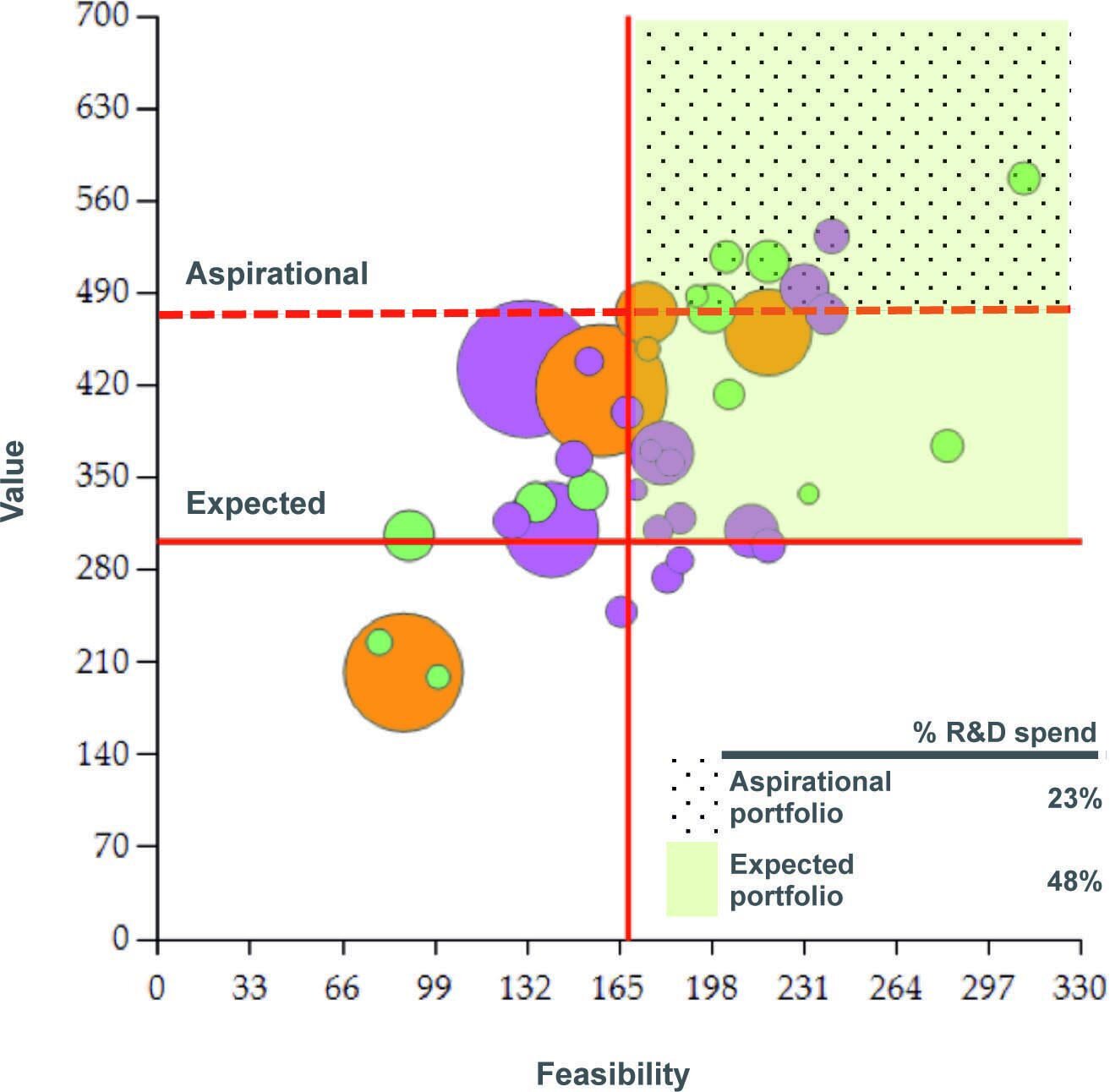
How smart pharma can stop making bad R&D choices
R&D portfolio strategy is so … unstrategic. Together with Navigant’s Greg Belogolovsky, we have some ideas to fix it.
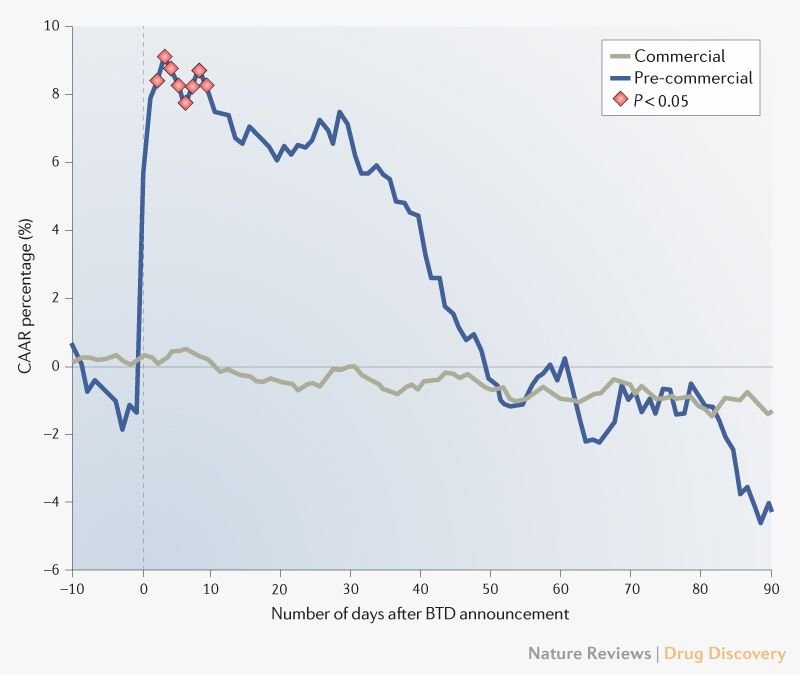
When biotechs get breakthrough therapy status, Mr. Market yawns
As Jay-Z might say, breakthrough status may provide 99 benefits, but excess stock returns ain’t one.

The cost-benefit revolution is far from over
In his new book, Cass Sunstein explores how to bring analytic rigor to policy decisions.

How to prevent drug price shenanigans
Drug price scandals aren’t all the same - but here’s one that may be preventable.
